Abstract
Background: The prognosis of TP53 mutated acute myeloid leukemia (AML) is dismal. Due to their correlation with universally bad outcomes, TP53 mutations play a crucial role in risk assessment and treatment selection in 5-10% of AML cases. Traditional Therapies have shown limited response and poor overall survival (OS). With current therapy, the median OS of a patient with newly diagnosed TP53m-AML is 6-9 months, and only around 10% of patients survive three years after allogenic hematopoietic stem cell transplantation (alloHCT). We present the safety and efficacy of novel therapeutic agents that target specifically TP53 mutated AML.
Materials & Method:
A comprehensive literature search was performed on PubMed, Cochrane Library, Embase, and Web of Science. We used the following mesh terms and Emtree terms, "acute myeloid leukemia" OR "AML" AND "TP53 mutations" from the inception of literature till 06/01/2022. We screened 2300 articles and included articles citing novel therapies such as "Venetoclax" (VEN), "Hypomethylating agents (HMA), 5-azacytidine (5-AZA), "checkpoint inhibitors, and "MDM2 inhibitors". We extracted data for "complete response (CR), Overall Survival (OS), and Progression-free survival (PFS) and safety variables such as grade III-IV adverse events. We excluded case reports, case series, preclinical trials, single-arm studies, review articles, meta-analyses, and controlled. STATA version 14.2 was used.
Results:
A total of 1814 patients were included in three-phase III and eleven phase II trials.
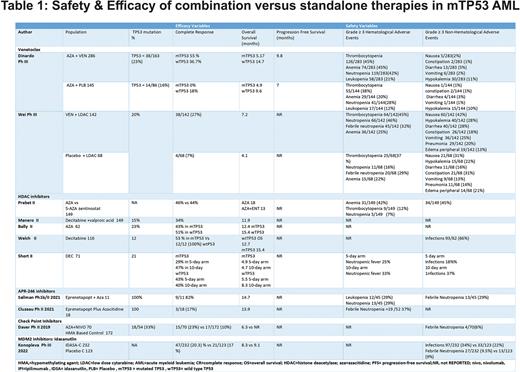
Bcl-2 inhibitors :For VEN in combination with two phase III trials incorporating 641 patients were evaluated; In both trials, encouraging remission rates were reported in de-nov or relapsed or refractory patients harboring TP53 mutations. However, the median OS was 14.7 vs 5.17 (mTP53 vs wTP53) months in the treatment group compared to 4.9 vs 9.6 (mTP53 vs wTp53) months in the control group respectively. For VEN +AZA combination group, CR rates in mTP53 patients was 55% versus 0% in AZA alone (p < 0.001). VEN with low-dose cytarabine (LDAC) had 27% CR, whereas LDAC alone had 7%.
Checkpoint inhibitors such as nivolumab showed favorable responses, as in the phase II study of nivolumab in combination with azacitidine in relapsed/refractory (R/R) AML; it demonstrated acceptable toxicity and an overall response rate (ORR) of 33%. However, increased response rates were seen in HMA-naïve patients.
HDAC inhibitors : In two phase II trials incorporating AZA vs AZA + entinostat, the CR was 46% vs 44% . The mOS achieved in AZA was 18 months vs. combination therapy with entinostat. In the study by Manero et al. , the combination therapy of Decitabine and valproic acid showed CR of 34% and mOS of 11.9 months. The results of other trials with respect to TP53 mutation is shown in table 1.
APR-246 inhibitors: In two phase II trials combining Eprentapopt with AZA , 29 patients were enrolled and CR of 41% was achieved.Sallman et al. described a remission of 82% with a median OS of 14.7 months in Eprenetapopt + 5-AZA. The most common grade ≥ 3 hematological adverse events were anemia, neutropenia, thrombocytopenia, and leukopenia.
MDM2 Inhibitors : In the MIRROS trial, a phase III trial incorporating idasanutlin + cytarabine + placebo + cytarabine in R/R AML;436 patients were enrolled. The CR was 20.3%vs 17.1%(odds ratio [OR], 1.23; 95%CI, 0.70-2.18). Common any-grade adverse events (≥ 10%incidence in any arm) were diarrhea (87.0%vs 32.9%), febrile neutropenia (52.8%vs 49.3%), and nausea (52.5%vs 31.5%).
The grade ≥3 hematological and non-hematological adverse events have been reported in Table 1.
Conclusion:
Emerging therapies employ unique therapeutic pathways that have led to increased optimism regarding the therapeutic potential of TP53 mutations. Although the median OS remains approximately a year with these therapies, hypomethylating drugs in combination with VEN or as independent therapy demonstrated greater response rates than induction chemotherapy. Initial progress has resulted from eprenetapopt's direct targeting of mutant p53 protein, showing a favorable 82% complete response. Still a daunting challenge, a combination of these therapies may ultimately be necessary to obtain adequate and sustained responses. Participation in clinical trials for all newly diagnosed AML patients should be encouraged to promote the development of innovative therapeutics.
Disclosures
Anwer:Janssen: Consultancy; Allogene Therapeutics: Research Funding; BMS: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal